What is s block elements Notes Class 11?
The S block elements is a chapter of chemistry in Class 11 of NCERT books. The chapter highlights the elements of the first two groups of the periodic table, which are collectively known as the s block elements.
What elements are in S block?
The elements in which the last electron enters the outermost s-orbital, belong to the s-block of elements. As the s-orbital can accommodate only two electrons, two groups (1 & 2) belong to the s-block of the periodic table.
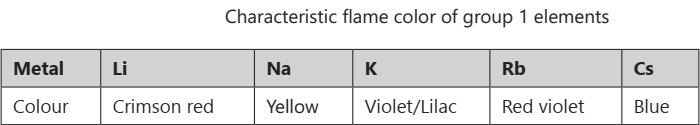
Group 1 of the periodic table consists of the elements: lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs) and francium (Fr).
Collectively known as the alkali metals, because they form hydroxides on reaction with water, which are strongly alkaline in nature.
What are alkaline earth metals and why are they called so?
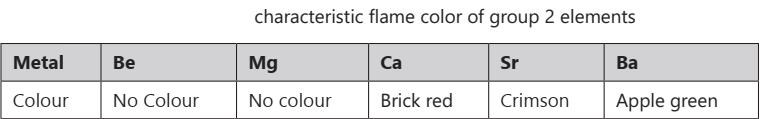
The elements of Group 2 include beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba) and radium (Ra). These elements with the exception of beryllium are commonly known as the alkaline earth metals. These are so-called because their oxides and hydroxides are alkaline in nature and these metal oxides are found in the earth’s crust.
TRENDS IN PHYSICAL AND CHEMICAL PROPERTIES
What is the electronic configuration of S block elements?
All alkali metals have one valence electron, ns1 outside the noble gas core. The loosely held s-electrons in the outermost valence shell of these elements make them the most electropositive metals. They readily lose electrons to give monovalent M+ ions. Hence, they are never found in the free state in nature.
Alkaline earth metals have two electrons in the s-orbital of valence shell. The compounds of these elements are also predominantly ionic.

Atomic and Ionic Radii
The alkali metal atoms have the largest size in a particular period of the periodic table. The atomic and ionic radii of alkali metals increase down the group i.e., they increase in size while going from Li to Cs. Due to the increased nuclear charge in these elements, the atomic and ionic radii of the alkaline earth metals are smaller than those of the corresponding alkali metals in the same periods. Within the group, the atomic and ionic radii increase with the increase in atomic number due to the increase in number of atomic shells.
Ionization Enthalpy
The ionization enthalpies of the alkali metals are considerably low and decrease down the group from Li to Cs. This is because the effect of increasing size outweighs the increasing nuclear charge and the outermost electron is very well screened from the nuclear charge.
The alkaline earth metals have low ionization enthalpies due to the fairly large size of the atoms. Since the atomic size increases down the group, their ionization enthalpy decreases.
Physical Properties Of S Block Elements:
All alkali metals are silvery-white, soft and light metals. This is due to having only one valence electron which participates in bonding. These elements have a low density which increases down the group from Li to Cs, because of their larger size. However, potassium is lighter than sodium because of its larger atomic volume.
The melting and boiling points of the alkali metals are low, which indicate weak metallic bonding, due to the presence of only a single valence electron in them. The strength of the metallic bond decreases down the group, and the m.p. decreases accordingly.
The m.p of lithium is nearly twice as high as that of sodium because of the stronger metallic bonding on account of its smaller size. However, the m.p. of all the others are close together.
The alkali metals and their salts impart a characteristic colour to oxidizing flames. This is because the heat from the flame excites the outermost orbital electron to a higher energy level.
CHEMICAL PROPERTIES OF S BLOCK ELEMENTS
Alkali metals are highly reactive due to their larger size and low ionization enthalpy. The reactivity of these metals increases down the group. The alkaline earth metals are less reactive than the alkali metals. The reactivity of these elements increases on going down the group.
Reasons for High Reactivity of Alkali Metals:
Alkali metals exhibit very high chemical reactivity, and the reasons for their high reactivity are:
(a) Low IE,
(b) Low heat of atomization
(c) High heats of hydration
Reactivity towards Air and Water
Alkali metals tarnish in dry air due to the formation of their oxides which in turn react with moisture to form hydroxides. They burn vigorously in oxygen, forming oxides.
Lithium forms a monoxide, sodium forms a peroxide, and the other metals form a superoxide.
The superoxide O2– ion is stable only in the presence of larger cations such as K, Rb, Cs.
4Li + O2 → 2Li2O (oxide)
2Na + O2 → Na2O2 (peroxide)
M + O2 → MO2 (superoxide) (where M = K, Rb, Cs).
The alkali metals react with water to form hydroxides and dihydrogen. The reaction becomes increasingly violent on descending the group.
Although lithium the has most negative electrode potential (EΘ) value, its reaction with water is less vigorous than that of sodium which has the least negative electrode potential (EΘ) value among the alkali metals. This behaviour of lithium is attributed to its small size and very high hydration energy.
Other metals of the group react explosively with water.
2M + 2H2O → 2MOH + H2 (M = alkali metal cation).
They also react with proton donors such as alcohol, gaseous ammonia and alkynes. This is because of their high reactivity towards air and water, the alkali metals are normally kept in kerosene oil.
Beryllium and magnesium are inert to oxygen and water because of the formation of an oxide film on their surface.
Reactivity towards Dihydrogen
The alkali metals react with dihydrogen at about 673 K (lithium at 1073 K) to form hydrides. All the metal hydrides are ionic solids with high melting points.
All alkaline earth metals except beryllium combine with hydrogen upon heating to form their hydrides, MH2, which are also high melting solids.
BeH2 however, can be prepared by the reaction of BeCl2 with LiAlH4 .
2BeCl2 + LiAlH4 → 2BeH2 + LiCl + AlCl3
Reactivity towards Halogens
The alkali metals readily react vigorously with halogens to form ionic halides, MX. However, lithium halides are somewhat covalent. This is because of the high polarization capability of the lithium ion. The Li+ ion is very small in size and has a high tendency to distort electron clouds around the negative halide ion. Since anion with larger sizes can be easily distorted, among halides lithium iodide is the most covalent in nature.
All the alkaline earth metals combine with halogens at elevated temperatures forming their halides.
M + X2 → MX2 (X = F, Cl, Br, l)
Reducing Nature
Why S block elements are reducing agents?
Alkali metals, are strong reducing agents, lithium being the most and sodium the least powerful with the small size of its ion, lithium has the highest hydration enthalpy which accounts for its high negative EΘ value and its high reducing power.
Like alkali metals, the alkaline earth metals are strong reducing agents. This is indicated by the large negative value of their reduction potentials.
Beryllium has a less negative value compared to other alkaline earth metals, due to a relatively large value of the atomization enthalpy of the metal.
However, its reducing nature is due to a large hydration energy associated with the small size of Be2+ ion.
Solution in Liquid Ammonia
The alkali metals dissolve in liquid ammonia giving deep blue solutions.
M + (x + y) NH3 → [M (NH3)x]+ + [e (NH3)y ]–
In dilute solutions, the main species are metal ions (M+) and electrons, which are solvated (i.e. ammoniated). The blue colour, corresponding to a broad absorption band near 1500 nm that falls into the visible range, is attributed to the solvated electron.
The blue solution of alkali metals in liquid ammonia, decompose very slowly with the liberation of hydrogen (i.e., reduction of the solvent).
M+ (am) + e– (aq) + NH3 ( l ) → MNH2(am) + ½ H2 (g)
Where, ‘am’ denotes solution in ammonia.