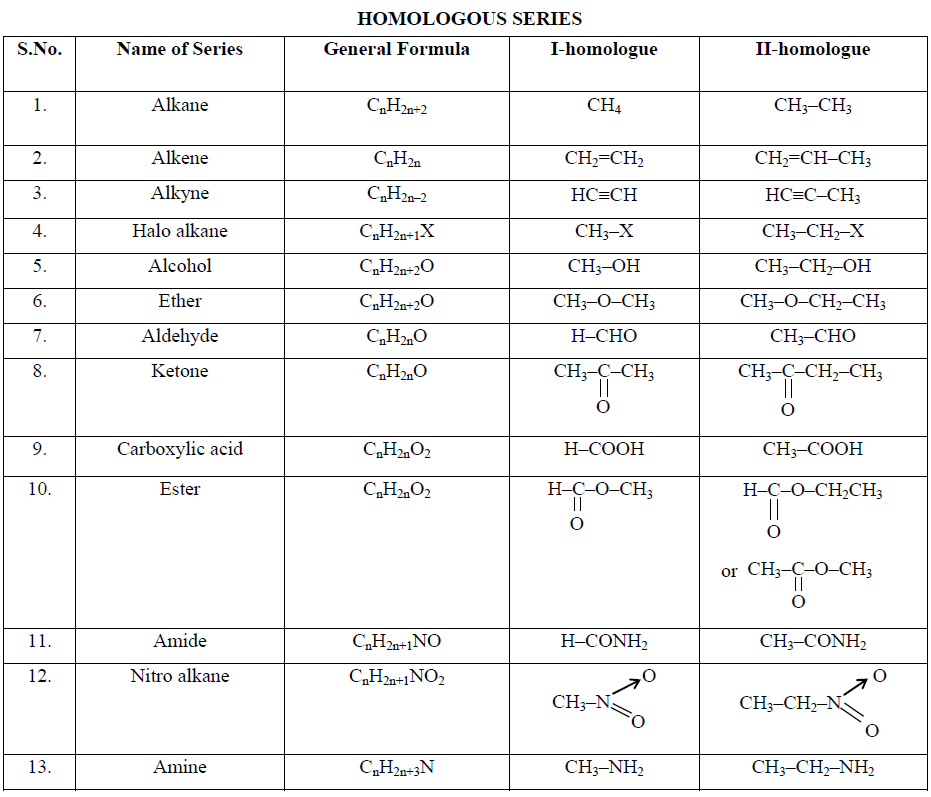
Organic compounds belonging to the main class can be divided into different families, known as homologous series, on the basis of similarity in structure and chemical properties. All compounds having the same carbon framework (skeleton) and the same functional group in their molecules possess similar properties. Such compounds when arranged in order of their molecular masses constitute a series called homologous series and the individual members are called homologues. The property by virtue of which a number of organic compounds form a homologous series is termed “homology”.
Definition
A homologous series (homos is Greek for “the same as”) can be defined as the group of compounds in which the various members have similar structural features and similar chemical properties and the successive members differ in their molecular formula by one methylene (CH2) group.
Characteristics Of Homologous Series
The general characteristics of a homologous series are:
(i) All compounds in the series are composed of the same elements and contain the same functional group.
(ii) All compounds in the series can be represented by one general formula, e.g., the homologous series of monohydric alcohols can be represented by the general formula CnH2n+1OH. The formula of various homologues can be written by giving the values 1, 2, 3, 4, ….. to n.
(iii) The molecular mass of every two adjacent members differs by 14 (CH2 = 12 + 2 x 1 = 14).
(iv) All compounds in the series have similar chemical properties because of the presence of the same functional group.
(v) The members of the series show a gradual gradation in their physical properties like solubility, density, melting and boiling points. The physical properties generally increase as the molecular mass increases.
(vi) The homologues can be prepared by almost similar methods. These are known as general methods of preparation.
There are a number of homologous series in organic compounds. Some important series of aliphatic compounds are listed below:
If we examine the unbranched alkanes, we notice that each alkane differs from the preceding alkane by one —CH2— group. Butane, for example, is CH3(CH2)2CH3 and pentane is CH3(CH2)3CH3. At room temperature (25°C) and 1 atm pressure, the first four members of the homologous series of unbranched alkanes are gases, the C5—C17 unbranched alkanes (pentane to heptadecane) are liquids, and the unbranched alkanes with 18 and more carbon atoms are solids.