DL Nomenclature is the most commonly used system for assigning the configuration to be given enantiomer. This d/l nomenclature system is based upon the comparision of the projection formula of one enantiomer to which the name is to be assigned, with that of a standard substance arbitrarily chosen for comparision.
The following two conventions are used for this purpose:
- Hydroxy Acid or Amino Acid Convention
- Sugar Convention
Hydroxy Acid or Amino Acid Convention
According to this convention, the prefix D- and L- refer to the configuration of the lowest numbered chirality centre of α-hydroxy or α-amino acids in the Fischer projection formula. If the α-OH or α-NH2 group is on the right-hand side (of the viewer), the prefix D- is used, whereas if these groups are on the left-hand side, the prefix L- is used.
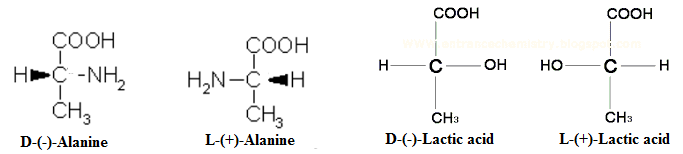
Sugar Convention
This convention was evolved by Emil Fischer who arbitrarily assigned D- and L- configurations to (+) and (-)-glyceraldehydes, respectively, as they were dextrorotatory and laevorotatory. He further assigned D-configuration (OH on the right) to (+)-glyceraldehyde and L-configuration (OH on the left) to (-)-glyceraldehyde, without any evidence.
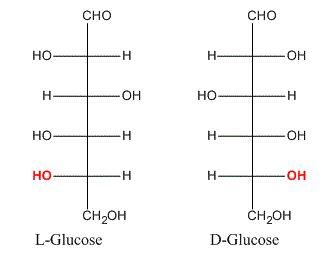
The relative configurations of a large number of compounds were determined by correlating them with D-(+)- or L-(-)-glyceraldehyde, e.g. relative configuration of (-)-lactic acid was designated as D-(-)-lactic acid as it had the same configuration as D-(+)-glyceraldehyde. For compounds containing several chiral carbon atoms, the configuration at one centre is related to glyceraldehyde, and the configurations of other carbon atoms are determined relative to that carbon. In the case of glucose, this carbon atom is C5, which is next to the CH2OH group, i.e. the highest numbered chiral carbon. Since naturally occurring glucose was assumed to have the OH group at C5 designated D-glucose. In case of the compounds having the OH group on the highest numbered chiral carbon on the left side, notation L is used.
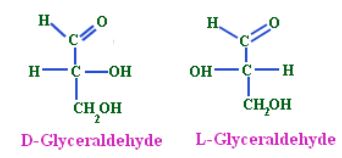
The relative configurations of a large number of compounds were determined by correlating them with D-(+)- or L-(-)-glyceraldehyde, e.g. relative configuration of (-)-lactic acid was designated as D-(-)-lactic acid as it had the same configuration as D-(+)-glyceraldehyde. For compounds containing several chiral carbon atoms, the configuration at one centre is related to glyceraldehyde, and the configurations of other carbon atoms are determined relative to that carbon. In the case of glucose, this carbon atom is C5, which is next to the CH2OH group, i.e. the highest numbered chiral carbon. Since naturally occurring glucose was assumed to have the OH group at C5 designated D-glucose. In case of the compounds having the OH group on the highest numbered chiral carbon on the left side, notation L is used.
In 1951, however, Bijvoet determined the absolute configuration of (+)-sodium rubidium tartarate using X-ray crystallography. It was found to be the same as assigned by Fischer. Before Bijvoet’s work, the configuration of thousands of compounds was assigned relative to (+)-tartaric acid. As a result of this finding, all the relative configurations of a large number of compounds became their absolute configurations.
Limitations of the Sugar Convention
(i) Sugar molecules have more than one chirality centre. The configuration of only the highest numbered chirality centre is assigned, and that of the other centres are not shown (hidden in their names).
(ii) Another complication is encountered when compounds of one series are converted into the other series. For example, saccharic acid, which is obtained by the oxidation of D-glucose, is assigned D-configuration as the configuration of the highest numbered chiral carbon is D. Now, if we rotate the molecule by 180o in the plane of the paper we get formula 25, which should be designated as L. This is a very serious drawback in the system, i.e. the same molecule can have both D- and L- configurations.
(iii) (+)-Tartaric acid and (-)-threonine can be designated as D as well as L depending upon whether the reference compound is glyceraldehyde (highest numbered chiral carbon) or hydroxyl or amino acid (lowest numbered chiral carbon). In conclusion, it may be added that this system suffers from several drawbacks, and is of limited use as it is confined only to sugars, hydroxy acids and amino acids.
This is just the basic concept on the D/L Nomenclature with few examples. A lot more is there to be discussed, which we shall learn in our later articles.