In this unit, we will be discussing what Octet Rule is and what are its limitations and what Sidgwick Powell theory is all about.
What is Octet Rule with example?
Here we will learn the basics of the Octet rule along with some examples so we can understand the topic better.
The Lewis theory was the first explanation of a covalent bond in terms of electrons that was generally accepted. If two electrons are shared between two atoms, this constitutes a bond which binds the atoms together. When the atom is surrounded by eight electrons in case of many light atoms, a stable arrangement is attained. This octet can be made up of some electrons which are ‘totally owned’ and some electrons which are ‘shared’. Thus atoms continue to form bonds until they have made up an octet of electrons. This is called the ‘octet rule’.
There are exceptions to the octet rule; for example, hydrogen is stable with only two electrons.
Octet Rule Of Cl2
A chlorine atom has seven electrons in its outer shell, so by sharing one electron with another chlorine atom both atoms attain an octet and form a chlorine molecule Cl2.

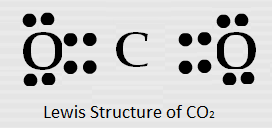
A carbon atom has four electrons in its outer shell, and by sharing all four electrons and forming four bonds it attains octet status in CCl4.
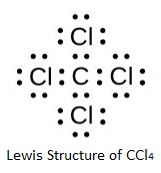
In a similar way, a nitrogen atom has five outer electrons, and in NH3 it shares three of these, forming three bonds and thus attaining an octet. Hydrogen has only one electron, and by sharing it attains a stable arrangement of two electrons.
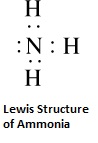
In a similar way, an atom of oxygen attains an octet by sharing two electrons in H2O and an atom of fluorine attains an octet by sharing one electron in HF.
Double bonds are explained by sharing four electrons between two atoms, and triple bonds by sharing six electrons.
Limitations Of Octet Rule
We have several Limitations of Octet Rule. So we are going to discuss it in this part of the unit with some examples.
The drawbacks of octet rule are as follows:
- For example, atoms such as Be and B which have less than four outer electrons. Even if all the outer electrons are used to form bonds an octet cannot be attained.
- The octet rule is also broken where atoms have an extra energy level which is close in energy to the p level and may accept electrons and be used for bonding. PF3 obeys the octet rule, but PF5 does not. PF5 has ten outer electrons and uses one 3s, three 3p and one 3d orbital. Any compound with more than four covalent bonds must break the octet rule. These violations become increasingly common in elements after the first two periods of eight elements in the periodic table.
- The octet rule does not work in molecules which have an odd number of electrons, such as NO and CIO2, nor does it explain why O2 is paramagnetic and has two unpaired electrons.
What is Sidgwick Powell theory?
Another theory called the Sidgwick Powell Theory gives us some information regarding the shape of the molecules.
In 1940 Sidgwick and Powell reviewed the structures of molecules then known. They suggested that for molecules and ions that only contain single bonds, the approximate shape can be predicted from the number of electron pairs in the outer or valence shell of the central atom. The outer shell contains one or more bond pairs of electrons. But it may also contain unshared pairs of electrons (lone pairs). Bond pairs and Ione pairs were taken as equivalent since all electron pairs take up some space, and since all electron pairs repel each other. Repulsion is minimized if the electron pairs are orientated in space as far apart as possible.
- If there are two pairs of electrons in the valence shell of the central atom, the orbitals containing them will be oriented at 180° to each other. It follows that if these orbitals overlap with orbitals from other atoms to form bonds, then the molecule formed will be linear.
- If there are three electron pairs on the central atom, they will be at 120° to each other, giving a plane triangular structure.
- For four electron pairs, the angle is 109028′, and the shape is tetrahedral.
- If there are five pairs of electrons, the shape is a trigonal bipyramid.
- For six pairs the angles are 90° and the shape is octahedral.