This article is on the Hydrocarbons Class 11 Notes of Chemistry. The notes on Hydrocarbons of class 11 chemistry have been prepared with great care keeping in mind the effectiveness of it for the students. This article provides the revision notes of the Hydrocarbons chapter of Class 11 for the students so that they can give a quick glance at the chapter.
The chapter has been divided into two articles. This article (Part 1) is on Alkanes. The second article (Part 2) is on Alkenes and Alkynes.
Hydrocarbons Part 1
Classification of Hydrocarbon
Hydrocarbon – Molecule with only C – C and C – H bonds
Alkane – Hydrocarbons with only single bonds.
Alkene – Hydrocarbons with one or more double bonds.
Alkyne – Hydrocarbons with one or more triple bonds.
Saturated Hydrocarbons or Alkanes or Paraffins
- Alkanes have general formula CnH2n+2 where n ranges from 1 to n.
E.g., CH4 – Methane , C2H6 – Ethane, C3H8 Propane
- Alkanes are not very reactive by nature and are hence also called paraffin. (Latin: Parum = little, affins = affinity)
- Each carbon atom is sp3 hybridized and its four bonding orbitals are directed towards the four corners of a regular tetrahedron at an angle of 109o28’ or 109.5o.
Nomenclature and Structure of Alkanes

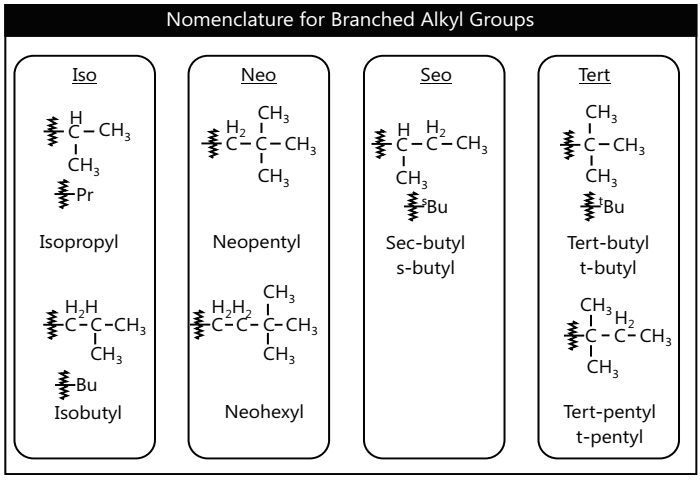
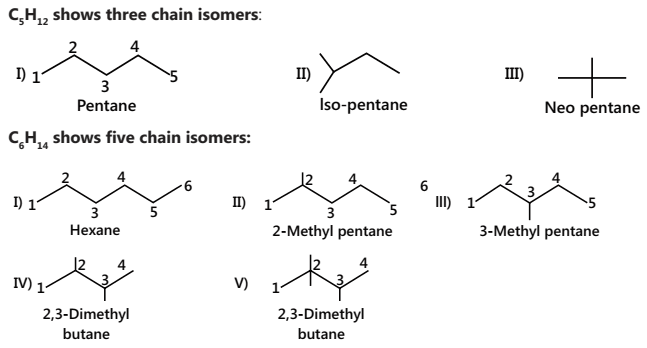
Isomerism in Alkanes
Isomers are structures with the same molecular formula but with a different atomic arrangement.

Constitutional isomers are isomers that differ in atom connectivity.
PREPARATION OF ALKANES
- From Unsaturated Hydrocarbons
a. Hydrogenation
Hydrogenation or reduction is the process by which hydrogen is added to an unsaturated compound in the presence of a catalyst like Pt, Pd or Ni.

Alkenes and alkynes add one and two molecules of hydrogen, respectively, in the presence of a catalyst, such as finely divided nickel or palladium or platinum at 523–573 K to form alkanes. This reaction is commonly referred to as Sabatier and Sanderson’s reduction.
Cycloalkane on hydrogenation gives alkane. The reactivity order of cycloalkanes is:
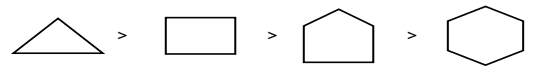
This is due to the angle strain. Greater the angle strain in the ring, less stable and more reactive is the compound. So, cyclopropane is the most reactive and gives propane on hydrogenation at a lower temperature, whereas higher cycloalkanes require high temperature.
- From Alkyl Halides
(i) Reduction of Alkyl Halide
RX + H2 → RH + HX
RX can be reduced by Zn + CH3COOH or Zn + dil. HCI or Zn + NaOH
(ii) Wurtz reaction
The reaction of an Alkyl halide with sodium in dry ether to give a hydrocarbon containing double the number of carbon atoms present in the halide.

- From Carboxylic Acids
(i) Decarboxylation
The process by which a molecule of CO2 is removed from an organic compound is called decarboxylation. When a carboxylic acid is heated with soda-lime (NaOH + CaO in the ratio 3: 1) at about 630K, a molecule of CO2 is lost and an alkane with one carbon atom less than the carboxylic acid is formed.
R – COOH + NaOH → R – COONa + H2O
R – COONa + NaOH – CaO, 630K → R — H + Na2CO3
NaOH alone could have been used in the above reaction but soda–lime is preferred because of the following two reasons:
(i) CaO permits the reaction to be carried out at a relatively higher temperature to ensure complete decarboxylation.
(ii) CaO keeps NaOH dry because it is quite hygroscopic (absorbs moisture from the air) in nature.
(ii) Kolbe’s Electrolysis
The electrolysis of an aqueous solution of sodium or potassium salt of a fatty acid gives a higher alkane, and the electrolysis of an aqueous solution of the sodium or potassium salt of dibasic acid gives alkene, whereas the electrolysis of aqueous solution of sodium or potassium salt of dibasic unsaturated fatty acid gives alkyne.
2RCOONa — Electrolysis → R – R + 2CO2 + 2OH- + H2
Mechanism (free radical):
2RCOONa — Electrolysis → 2RCOO– + 2Na+
At anode (oxidation)
2RCOO– — Electrolysis → 2RCOO• + 2e–
2R• + 2CO2 → R — R
At cathode (reduction): Reduction of H2O takes place since its reduction potential is greater than Na.
2H2O + 2e– → 2OH– + H2
PROPERTIES OF ALKANES
- Boiling points: In straight-chain alkane C1 to C4 are gases, C5 to C17 are liquids and C18 on words are colourless waxy solids. With an increase in the molecular size the surface area increases and hence Van der-Waals forces increase which increases B.P. accordingly. B.P. of branched-chain isomers is lower than corresponding n-alkanes due to the decreased surface area.
- Melting points: Melting points of alkanes also increase with the increase in carbon content but the variation is not regular. Alkanes with an even number of carbon atoms have higher m.p. than those with an odd number of carbons atoms. This property is commonly known as the alternation effect.
- Solubility: ‘Like dissolves like’ is the general rule of solubility. Non-polar alkanes which are insoluble in polar solvents such as water, alcohol, etc. are highly soluble in non–polar solvents such as petroleum ether, benzene, carbon tetrachloride, etc. greases are mixtures of higher alkanes and hence are non–polar and hydrophobic (water-repelling) in nature.
- Density: The densities of alkanes increase with an increase in the molecular masses until the limiting value of about 0.8g cm-3. This means that all alkanes are lighter than water.
REACTION OF ALKANES
Substitution Reactions
Halogenation
In this reaction, hydrogen of an alkane is replaced by halogen. Reaction proceeds by “Homolytic fission” in which free radicals are formed.
(i) Chlorination: During chlorination of methane, all the four hydrogen atoms are replaced one by one to form a mixture of products. For example,

Mechanism
Let’s look at the mechanism behind the substitution reaction of bromine reacting with methane.
Initiation: UV breaks the bond between Br2 leaving free radicals.

Propagation: Br reacts with methane, leaving another radical.
CH4 + Br• → •CH3 + HBr
(a) Propagation: The methyl radical reacts with another bromine molecule.
CH3• + Br – Br → CH3 Br + • Br
(b) Chain propagation: Each propagation step consists of two reactions. In the first reaction, the Cl radical attacks the CH4 molecule and abstracts a hydrogen atom forming •CH3 and a molecule of HCI as shown reaction (i) in the second reaction, •CH3 thus produced reacts further with a molecule of Cl2 forming a molecule of methyl chloride and another •Cl as shown in reaction (ii). The newly formed •Cl reacts with another molecule of CH4 (reaction (i)) to produce another molecule of HCl and another •CH3. This •CH3 can again repeat reaction (ii) and so on. Thus, the sequence of reactions in equations (i) and (ii) is repeated and the chain gets propagated.

When a sufficient amount of methyl chloride has been formed, the •Cl produced in reaction (ii) has a greater chance of colliding with a molecule of CH3Cl rather than a molecule of CH4. If such a collision occurs, a new free radical (•CH2Cl) is produced (reaction (iii)) which may subsequently react with Cl2 producing a molecule of CH2Cl2 (reaction (iv)) and another ·Cl. This process continues till all the hydrogen atoms of methane are replaced by halogen atoms (reactions (v), (vi), (vii) and (viii)).

Termination: Two radicals react and complete the reaction. Lots of Bromine will mean more substitution (dibromo, tribromo, etc.)
Br• + Br• → Br2
CH3• + Br• → CH3Br
CH3• + CH3• → C2H6
Combustion
In presence of sufficient oxygen, alkane gives water and carbon dioxide as products. The complete combustion reaction of butane is:
2C4H10(g) + 13O2(g) → 8CO2(g) + 10H2O(g) ∆H = –5754 kJ mol/L
In general, the formula for complete combustion of alkanes is:
CxHy (g) + (x + y/4) + O2(g) → xCO2(g) + y/2 H2O(g)
In the insufficient supply of O2 by incomplete combustion, carbon monoxide and elemental carbon are formed.
Incomplete combustion of octane: 2C8H18(l) + 17O2(g) → 16CO(g) + 18H2O(g)
Catalytic oxidation
(i) When CH4 and O2 (9: 1) are heated at 100 atm pressure and passed through copper wires at 470K, CH4 is oxidized to CH3OH.
2CH4 + O2 → 2CH3OH
(ii) CH4 and O2, when heated with molybdenum oxide, give methanal.
CH4 + O2 — ∆/MoO2 → HCHO + O2
(iii) Higher alkane (C16) on oxidation with manganese acetate at 370–430 K produces higher fatty acids.
2C16H34 + 3O2 — ∆/(CH3COO)2Mn → 2C15H31COOH + 2H2O
(iv) With alkaline KMnO4, 3OH atom is oxidized to (–OH) group.
Because of the +I effect of the three CH3 groups, e– density at 3OC atom is relatively high, which accounts for its easier oxidation.
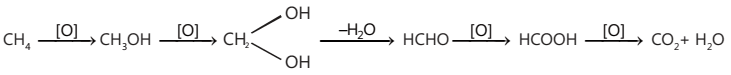
Isomerization
Alkanes on heating with AlCl3 at 570 K isomerize to give branched-chain alkane [takes place via Me shift].

Aromatization
Alkanes containing six to eight carbon atoms when heated to about 773 K under 10–20 atm pressure in the presence of a catalyst consisting of oxides of chromium, vanadium and molybdenum supported over alumina, get converted into aromatic hydrocarbons. This process which involves cyclization, isomerization and dehydrogenation is called aromatization. For example

Under similar conditions, n-heptane gives toluene while n-octane gives a mixture of o–, m– and p–xylenes.
Pyrolysis
Pyrolysis of higher alkanes to give a mixture of lower alkanes, alkenes, etc. is called cracking. It is usually carried out by heating higher alkanes to high temperatures (773–973 K) under a pressure of 6–7 atmospheres in the presence or absence of a catalyst. For example

Pyrolysis of alkanes involves the breaking of C – C and C – H bonds and occurs by a free radical mechanism. The preparation of oil gas from kerosene oil and petrol gas from petrol is based upon the process of pyrolysis. For example, dodecane, a constituent of kerosene oil, on heating to 973 K in the presence of Pt, Pd or Ni, gives a mixture of heptane and pentene along with other products.
Conformational Isomers
The infinite number of spatial arrangments that a molecule can adopt in space due to rotation about σ bonds are called rotational or conformational isomers. Analysis of energy changes during these rotations is called conformational analysis.
Check Part 2 of the Chapter Here: PART 2 – ALKENES AND ALKYNES
This article has tried to highlight all the important parts of Hydrocarbons in the form of short notes for class 11 students in order to understand the basic concepts of the chapter. The notes on Hydrocarbons have not only been prepared for class 11 but also for the different competitive exams such as IIT jee, neet, etc.