One of the important properties of actinides is the electronic configuration of actinides. The general electronic configuration of actinides is [Rn] 5f 0-14 6d 0-2 7s 2. The elements from atomic number 90 (Thorium) to atomic number 103 (Lawrencium) are called actinide elements. However, the electronic configuration of the actinides does not follow the simple pattern found in the lanthanides.
The electronic configuration of actinides is listed in the table below.
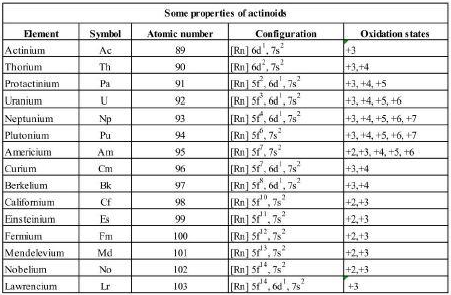
Examination of the preceding elements in the periodic table shows that Francium (Fr) and Radium (Ra) belong to Groups 1 and 2 and their outermost electrons must be in 7s orbitals. The next element Actinium Ac begins to fill the penultimate d shell (6d1 7s2).
The 5f and 6d subshells penetrate the 7s orbital quite significantly and are therefore stabilized relative to the 7p subshell. The next few electrons enter either of these two subshells, i.e., 5f and 6d. As we fill electrons from actinium onwards, the energies of 6d and 5f subshell don’t vary significantly and the allocation of exact configuration becomes difficult.
An important point is that around actinium both 6d and 5f subshells are of very similar energies, but as we keep filling the electrons the 5f subshell definitely acquires greater stability. From Uranium onwards, there is no doubt that the 5f subshell is more stable.