Metal to Ligand Charge Transfer (MLCT) transition is the transfer of an electron from an orbital with primarily
metal character to one with primarily ligand character. Since electron transfer from metal to ligand corresponds
to metal oxidation and ligand reduction, an MLCT transition occurs when a ligand that is easily reduced is bound to a metal centre that is readily oxidized.
Metal to Ligand Charge Transfer (MLCT) transitions are favoured in complexes in which
- Metal has a low oxidation state.
- Metal d-orbitals are filled.
- Metal d-orbitals are of relatively high energy.
- Ligands have empty π-antibonding orbitals.
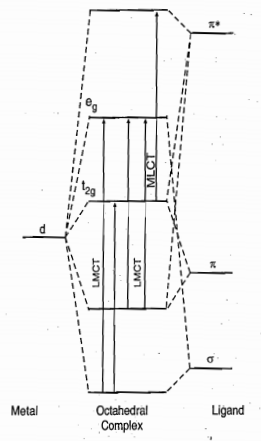
Metal to Ligand Charge Transfer (MLCT) mainly occurs with the ligands having π* orbitals such as CO, CN–, SCN–, pyridine, bipyridine, o-phenanthroline, pyrazine, dithioline, NO, etc.
In an octahedral complex when t2g and eg* orbitals (both belong to metal) are occupied, two MLCT bands π* ← t2g and π ← eg* are observed (π* is the vacant orbital of the ligand). If either t2g or eg* orbitals are occupied, then only one charge transfer band π* ← t2g or π ← eg* is observed.
Often, the associated absorption occurs in the UV region of the spectrum and is not responsible for producing intensely coloured species. In addition, for ligands where a ligand-centred π* ← π transition is possible (e.g. heterocyclic aromatics such as bpy), the MLCT band may be obscured by the π* ← π absorption.
For [Fe(bpy)3]2+ and [Ru(bpy)3]2+, the MLCT bands appear in the visible region at 520 and 452 nm, respectively. These are both metal(II) complexes, and the metal d-orbitals are relatively close in energy to the ligand π* orbitals, giving rise to an MLCT absorption energy corresponding to the visible part of the spectrum.
Related Questions
Why is KMnO4 Purple in Colour?
What is the reason for the colour of potassium dichromate?
What is the difference between dd transition and charge transfer transition?